3 unstable releases
| 0.2.0 | Jan 6, 2025 |
|---|---|
| 0.1.1 | Nov 25, 2024 |
| 0.1.0 | Nov 25, 2024 |
#74 in Science
181 downloads per month
Used in interatomic
240KB
2.5K
SLoC

Coulomb
A Library for Electrolyte Solutions and Electrostatic Interactions
Features
Coulomb is a library for working with electrolyte solutions and calculating electrostatic interactions in and between molecules and particles. The main purpose is to offer support for molecular simulation software.
- Temperature dependent dielectric permittivity models for common solvents.
- Handling of ionic strength and the Debye screening length.
- Automatic stoichiometry deduction for arbitrary salts.
- Extensive library of truncated electrostatic interaction schemes such as
Wolf, Reaction field, Real-space Ewald, generalized through a short-range function
trait. - Ewald summation with and without implicit salt.
- Multipole expansion for energies, forces, fields between ions, dipoles, and quadrupoles.
- Extensively unit tested and may serve as reference for other implementations or approximations.
- Partial support for static unit of measure analysis via
uom. To enable, use theuomfeature flag.
This is largely a Rust rewrite and extension of the CoulombGalore C++ library.
Examples
Dielectric Media and Electrolytes
Simple polynomial models are provided to obtain the relative permittivity or a Medium as a function
of temperature.
For working with the ionic strength, Salt of arbitrary valency can be given and the
required stoichiometry is automatically worked out.
use coulomb::{Medium, Salt};
let molarity = 0.1;
let medium = Medium::salt_water(298.15, Salt::CalciumChloride, molarity);
assert_eq!(medium.permittivity()?, 78.35565171480539);
assert_eq!(medium.ionic_strength()?, 0.3); // mol/l
assert_eq!(medium.debye_length()?, 5.548902662386284); // angstrom
Multipolar Interactions
All pairwise schemes support calculation of potential, energy, field, force from or between multipolar particles, up to second order (ion-ion, ion-dipole, dipole-dipole; ion-quadrupole). Most scheme can be evaluated with or without a Debye-Hückel screening length.
use coulomb::pairwise::*;
let scheme = Plain::default(); // Vanilla Coulomb scheme, 𝒮(𝑞)=1
let z = 1.0; // point charge, 𝑧
let r = Vector3::new(3.0, 5.0, 0.0); // distance vector, 𝒓
let ion_pot = scheme.ion_potential(z, r.norm()); // potential |𝒓| away from charge
assert_eq!(ion_pot, z / r.norm());
let mu = Vector3::new(0.2, 3.0, -1.0); // point dipole, 𝝁
let dipole_pot = scheme.dipole_potential(&mu, &r); // potential 𝒓 away from dipole
let energy = scheme.ion_dipole_energy(z, &mu, &r); // interaction energy assuming 𝒓 = 𝒓(𝜇) - 𝒓(𝑧)
The image below is generated by examples/potential.rs and shows the calculated
electric potential on a plane containing a monopole and a dipole.
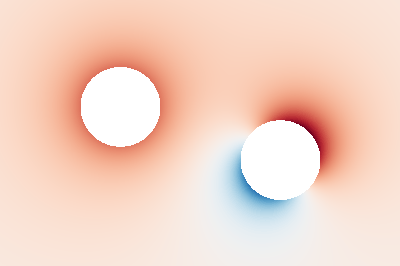
Unit analysis
Experimental support for static unit analysis can be activated with the uom feature.
use coulomb::{units::*, pairwise::{Plain, MultipoleEnergySI}};
let z1 = ElectricCharge::new::<elementary_charge>(1.0);
let z2 = ElectricCharge::new::<elementary_charge>(2.0);
let r = Length::new::<nanometer>(2.3);
let energy = Plain::without_cutoff().ion_ion_energy(z1, z2, r);
assert_eq!(energy.get::<kilojoule_per_mole>(), 362.4403242896922);
Dependencies
~5MB
~99K SLoC